Covid-19 Diagnostics
Abstract diagram narrative about data strategy
For the Covid-19 Diagnostics
Evidence Accelerator
The Covid-19 Diagnostics Evidence Accelerator is a multi-stakeholder, collaborative project leveraging real-world data in the diagnostic and antibody testing space.
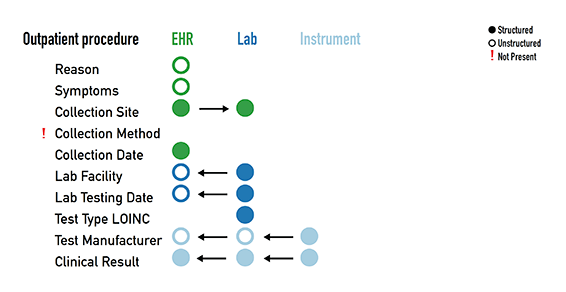
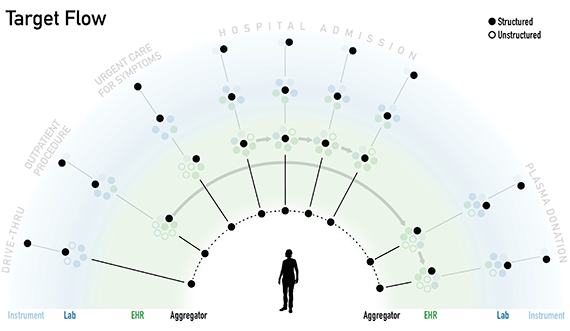
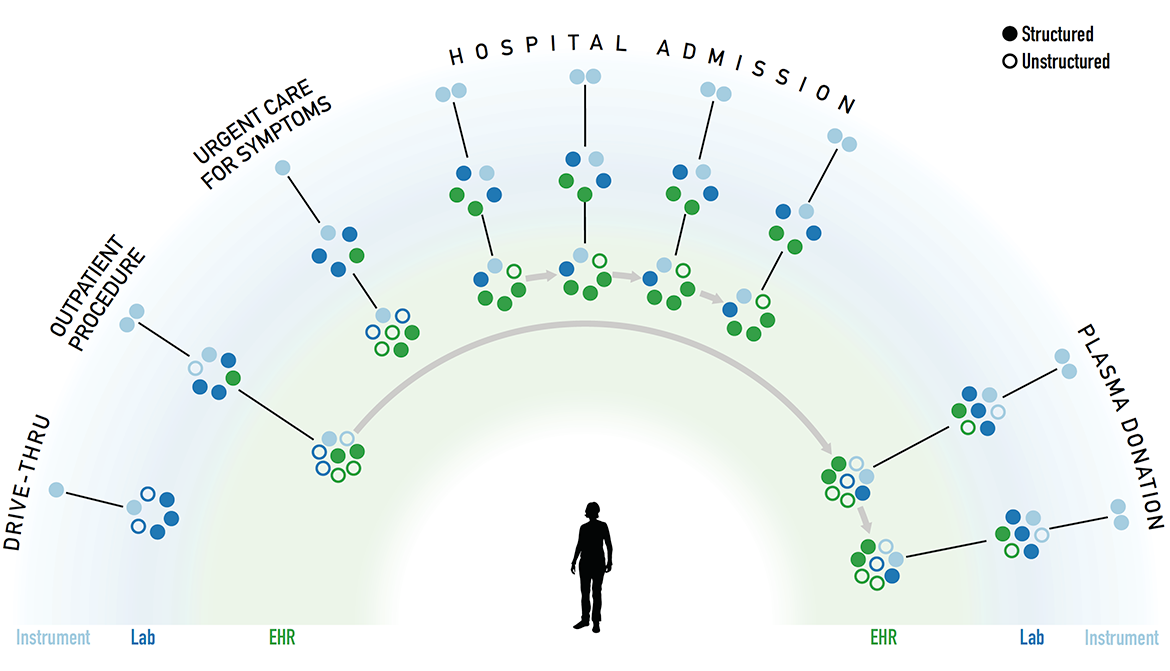
Shown are slides from an August 2020 presentation of an example Covid-19 patient diagnostic data story. The goal of the presentaiton was to depict data-connection goal and challenges. From the meeting notes:
At the instrument level, the data is structured. However, when the data moves into the EHR, there is a mixture of structured and unstructured data. There is no system that is capturing the full picture since there are different providers and systems that are involved in the data capture. This is the interoperability challenge.
Nearly 50 organizations participated in these meetings, including: Aetion, Ciox Real World Data, CSL Behring, Duke-Margolis Center for Health Policy, Flatiron Health, Health Catalyst, HealthVerity, Imperial College Health Partners, IQVIA, IVD Industry Connectivity Consortium, Lahey Hospital & Medical Center, Lilly Mayo Clinic, Medical Device Innovation Consortium, NorthWest EHealth Limited, Regenstrief Institute, Roche, UnitedHealth Group, and University of California Health System.